Digoxin Generics: Why Bioavailability Matters and How to Monitor Safety
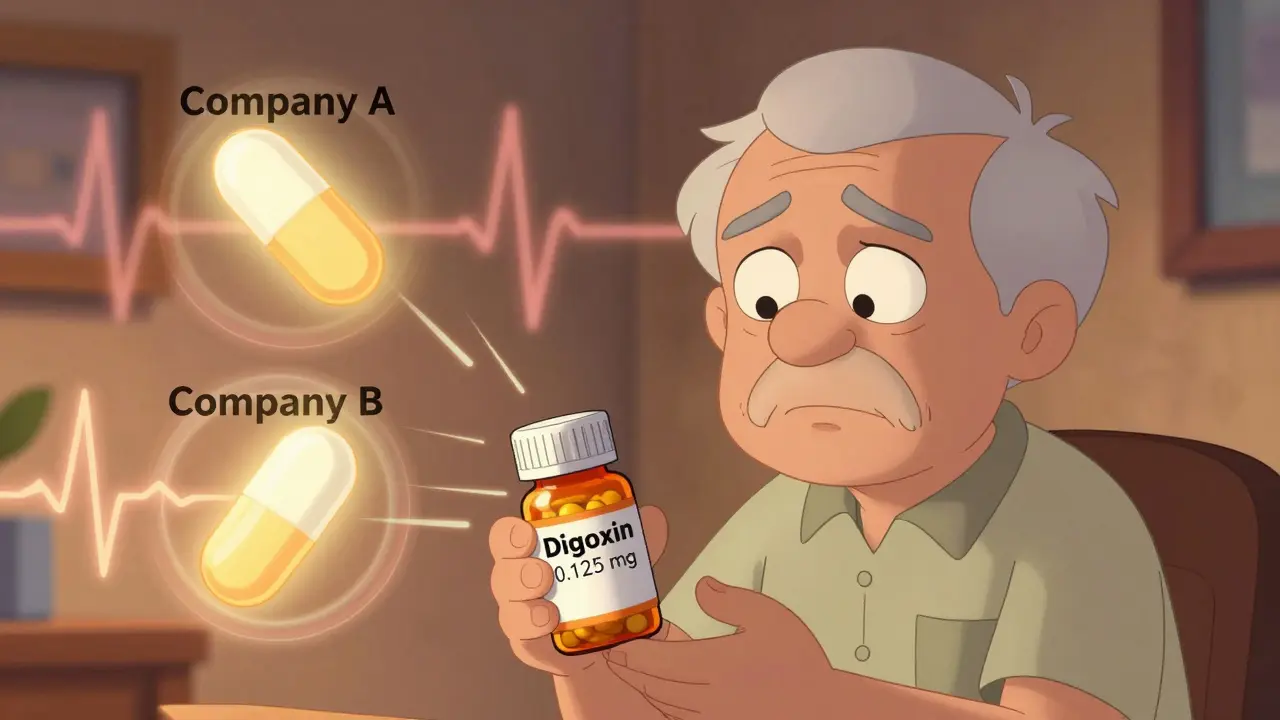
When you take digoxin, even a tiny change in how your body absorbs it can mean the difference between healing and hospitalization. This isn’t just another generic drug. Digoxin has a narrow therapeutic index - meaning the gap between a helpful dose and a dangerous one is razor-thin. The safe blood level? Between 0.5 and 2.0 ng/mL. Go a little over, and you risk life-threatening arrhythmias. Go a little under, and your heart failure or atrial fibrillation could worsen. That’s why switching between generic digoxin brands isn’t like switching from one brand of ibuprofen to another.
Why Digoxin Is Different
Digoxin has been around since the 1700s, derived from the foxglove plant. It’s not flashy, but it still saves lives - especially in older adults with heart failure or irregular heart rhythms. But here’s the catch: digoxin doesn’t play by the same rules as most other medications. The FDA treats it like a new drug, even for generics. Why? Because small differences in how much of the drug actually gets into your bloodstream can be deadly.
Most generic drugs must prove they’re bioequivalent to the brand name - meaning their absorption rate and total exposure (AUC) and peak concentration (Cmax) fall within 80-125% of the original. For digoxin, that’s the rule too. But here’s where it gets tricky: that 80-125% range is an average across a group of healthy volunteers. One person might absorb only 45% of the dose. Another might absorb 110%. The average could still meet FDA standards - but for that one person, the drop in absorption could mean their heart condition flares up.
And here’s the real problem: the FDA only tests one generic against the brand name (Lanoxin). They don’t test one generic against another. So if you’re stable on one generic - say, from Company A - and your pharmacy switches you to a different generic from Company B, you’re entering untested territory. No study has compared those two. But your body knows the difference.
What Bioavailability Really Means for You
Bioavailability is just a fancy way of asking: how much of the pill actually gets into your blood? For digoxin tablets, it’s usually around 60-80%. But that number can shift based on the filler, coating, or manufacturing process. Even minor changes in tablet compression or dissolution speed can alter absorption. And since digoxin has a long half-life - about 36 hours - any change sticks around. It doesn’t wash out quickly. That’s why a switch can quietly build up to toxicity over days.
One study in Estonia found that after confirming generic digoxin was bioequivalent to Lanoxin, use of the drug increased across the country. That’s good news - more people got access. But it also showed that once you start using a generic, you need to stay on it. Switching brands led to measurable changes in blood levels. In clinical reports, some patients saw their digoxin levels jump or drop by more than 25% after a switch. That’s not a rounding error. That’s a medical emergency.
Even the form matters. Digoxin elixir (liquid) is absorbed better than tablets - up to 70-85% of the IV dose. But if you’re on tablets and suddenly get switched to liquid without a dose adjustment, you could overdose. The same goes for switching from one tablet brand to another. The pill might look the same. The dose might say 0.125 mg. But your body doesn’t care what’s printed on it. It cares what’s actually getting in.
The Monitoring Checklist: What You Need to Do
If you’re on digoxin - whether brand or generic - you need a plan. Not just a prescription. A monitoring plan.
- Get a baseline level - 4 to 7 days after starting or changing your dose. This tells you where you’re starting.
- Test before every dose - Always draw blood right before your next scheduled dose (trough level). That’s when levels are lowest and most telling.
- Recheck after any switch - If your pharmacy changes your generic brand, get a blood test 3 to 5 days later. Don’t wait for symptoms.
- Test after any big change - If your kidney function drops, you start a new antibiotic, or you begin taking a diuretic, check your digoxin level. These all affect how your body clears the drug.
- Know the warning signs - Nausea, vomiting, blurry yellow-green vision, confusion, or a new irregular heartbeat? These aren’t just side effects. They’re red flags.
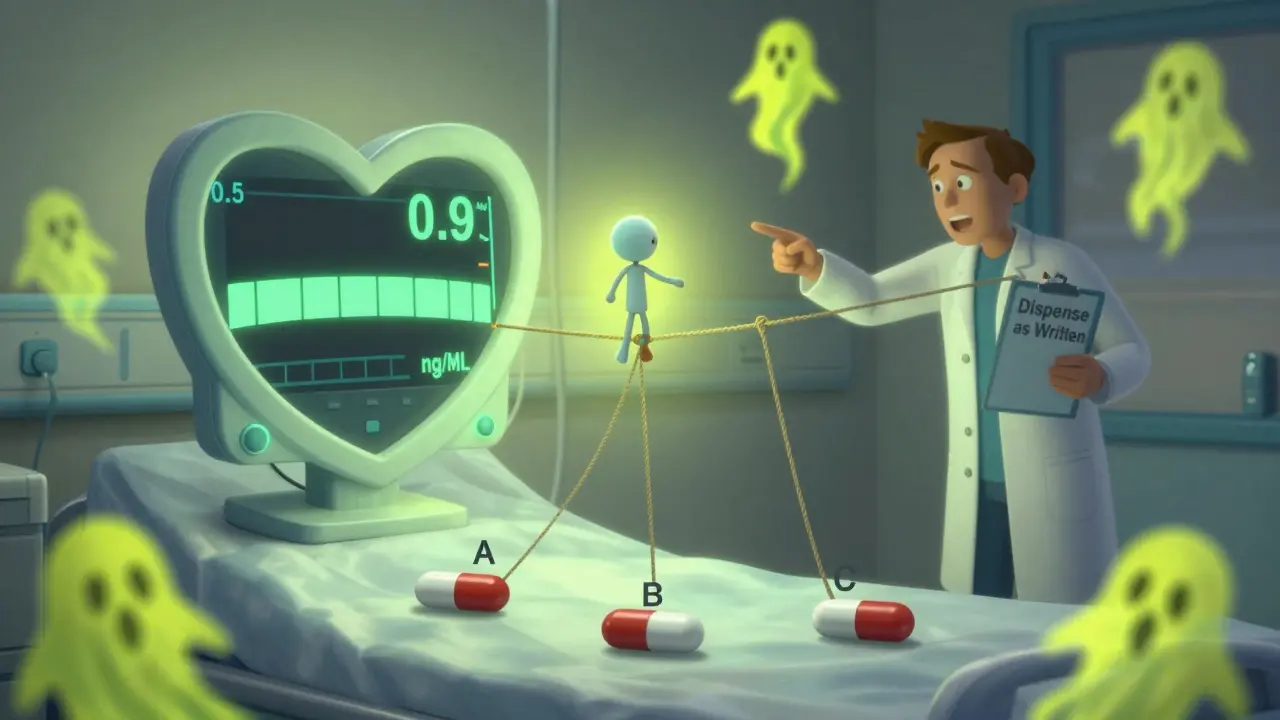
Recent evidence suggests the safest target range for heart failure patients is even lower: 0.5 to 0.9 ng/mL. Higher levels don’t mean better results - they just mean more risk. The American Heart Association and American College of Cardiology both say: stick with one manufacturer. If you’re stable, don’t switch.

Why Pharmacists Can’t Always Help
Pharmacists are trained to substitute generics. It’s standard practice. But digoxin isn’t standard. Most pharmacies don’t know the difference between one generic and another. They see two 0.125 mg tablets and assume they’re interchangeable. They’re not.
When a patient asks, “Can I switch to the cheaper one?” the pharmacist says yes - because legally, they’re allowed to. But they’re not required to know that digoxin is one of the few drugs where switching can kill. No one tells them. No one trains them. And no one checks if the patient’s doctor even knows about the switch.
The FDA lists three generic digoxin brands with an “AB” rating - meaning they’ve passed bioequivalence tests against Lanoxin. But that doesn’t mean they’re interchangeable with each other. It just means each one passed its own test against the brand. That’s not the same as being safe to swap.
What You Can Do Right Now
Here’s what you need to do - today.
- Check your prescription bottle. Who makes your digoxin? Write it down.
- Ask your pharmacist: “Is this the same manufacturer as last time?” If they say “yes,” ask them to confirm the name on the label. Don’t trust “it’s the same generic.”
- If you’ve switched brands in the last month, call your doctor. Ask for a digoxin blood test.
- Keep a log: date, dose, manufacturer, symptoms. Bring it to every appointment.
- If you’re over 65, have kidney disease, or take diuretics - be extra careful. Your body clears digoxin slower. Small changes hit harder.
There’s no magic solution. Generic digoxin is cheaper. That’s good. But cost savings shouldn’t come at the cost of safety. The same pill, from a different factory, can behave like a different drug.
What Doctors Should Be Doing
Doctors need to stop treating digoxin like a routine drug. They need to:
- Prescribe the manufacturer - not just the dose. Write “Dispense as written” or specify the brand name if possible.
- Set up automatic alerts in their EHR when a patient’s digoxin manufacturer changes.
- Recheck levels after every switch - no exceptions.
- Teach patients to recognize toxicity signs. Don’t assume they know.
There’s no excuse for letting patients slip through the cracks. Digoxin has been studied for decades. We know the risks. We know how to prevent them. But too many people are still being switched without warning.
Bottom Line: Stay Consistent, Stay Alert
Digoxin isn’t a drug you can afford to gamble with. Even if your generic is FDA-approved, even if your lab says you’re “in range,” your body might be quietly drifting toward danger. The science is clear: bioequivalence at the population level doesn’t guarantee safety at the individual level. And when you’re taking a drug where the difference between life and death is measured in nanograms per milliliter, that’s not a risk you can take.
Stick with one manufacturer. Know your dose. Know your levels. Speak up if something feels off. Your heart is counting on it.
Are all generic digoxin products the same?
No. While each generic must prove bioequivalence to the brand-name Lanoxin, they are not tested against each other. Switching between different generic manufacturers can cause unpredictable changes in blood levels, even if both are FDA-approved. This is especially dangerous because digoxin has a narrow therapeutic index - small changes can lead to toxicity or treatment failure.
How often should digoxin levels be checked?
Check digoxin levels 4 to 7 days after starting therapy or changing the dose. After switching to a different generic brand, recheck within 3 to 5 days. Also check levels if kidney function changes, new medications are added, or if you develop symptoms like nausea, vomiting, vision changes, or irregular heartbeat. Always draw blood just before your next dose (trough level) for the most accurate reading.
Can I switch from digoxin tablets to the liquid form?
Yes, but only under medical supervision. Digoxin elixir is absorbed more efficiently than tablets - up to 70-85% of the intravenous dose, compared to 60-80% for tablets. Switching without adjusting the dose can lead to overdose. Always consult your doctor before changing formulations, and get a blood level check after the switch.
Why is digoxin more dangerous in older adults?
Older adults often have reduced kidney function, which slows the clearance of digoxin from the body. This increases the risk of drug accumulation and toxicity. They’re also more likely to take other medications that interact with digoxin, like diuretics or antibiotics. Even small changes in absorption or clearance can push levels into the toxic range. For this reason, lower target levels (0.5-0.9 ng/mL) are now recommended for elderly patients with heart failure.
What should I do if my pharmacy switches my digoxin brand?
Don’t assume it’s safe. Contact your doctor immediately and ask for a digoxin blood level test 3 to 5 days after the switch. Watch for symptoms like nausea, vomiting, confusion, blurred vision, or a new irregular heartbeat. Keep a record of the manufacturer name on your prescription bottle and bring it to every appointment. If possible, ask your doctor to write “Dispense as written” to prevent future switches.






Juan Reibelo
January 23, 2026 AT 16:24Wow. I’ve been on digoxin for 8 years. Switched generics twice last year-both times, I got dizzy and my vision went yellow-green. Thought it was just aging. Turns out, it was the pill. Now I demand the same brand. My pharmacist thinks I’m nuts. I don’t care. I’m alive because I didn’t ignore it.
Write ‘dispense as written’ on the script. It’s not optional. It’s survival.
Sawyer Vitela
January 24, 2026 AT 21:40FDA’s AB rating is meaningless for digoxin. The 80-125% bioequivalence window is a statistical loophole. One person’s 75% absorption is another’s 110%. That’s not bioequivalence-that’s Russian roulette with a heart.
Study after study shows spikes in hospitalizations after generic switches. But regulators won’t act because it’s too expensive to test every combo. Profit over patients. Classic.
Tiffany Wagner
January 26, 2026 AT 09:49I’m a nurse and I see this all the time. Elderly patients get switched without a word. No warning. No follow-up. They come in confused, nauseous, and we have to dig through their pill bottles to even figure out which generic they’re on.
Doctors need to stop assuming pharmacists know this. We need a flag in the system. A red alert when digoxin changes hands.
Thank you for writing this. I’m printing it and handing it to my team.
Chloe Hadland
January 28, 2026 AT 06:10My grandma’s on digoxin. She’s 82, kidney issues, takes three other meds. We switched her to a cheaper generic last month because insurance said so. She got worse. Not dramatic, just… slower. Less alert. We didn’t connect it until I read this.
We got her tested. Level was 2.3. Toxic.
We switched back. She’s back to her self now. Thank you for making me look deeper.
Love you.
-
Amelia Williams
January 28, 2026 AT 11:02Okay, I’m not a doctor, but I’ve been researching this for my dad since he got switched. I found a study from 2021 in the Journal of Clinical Pharmacology that tracked 147 patients who switched generics. 68% had a >15% change in serum levels. 12% went into toxicity. And 3 of them were admitted.
Why isn’t this common knowledge? Why don’t we have a database? Why can’t pharmacies show the manufacturer on the label like they do with insulin?
I’m starting a petition. Anyone in? Let’s make this a thing.
Viola Li
January 29, 2026 AT 00:12So let me get this straight. You’re saying the FDA is lying to us? That generics aren’t safe? That we’re all lab rats for Big Pharma?
What’s next? That aspirin isn’t aspirin? That Tylenol is secretly laced with rat poison?
Wake up. This is just fearmongering dressed up as science. People get switched all the time. Most don’t die. You’re overreacting.
Dolores Rider
January 29, 2026 AT 03:28THIS IS A GOVERNMENT COVER-UP. 😱
They don’t want you to know that digoxin is being used to control the elderly population. The ‘toxicity’? It’s not the drug. It’s the tracking chip they put in the tablets. That’s why they won’t test generics against each other-they don’t want you to find the frequency.
My cousin’s neighbor’s dog got sick after eating a digoxin pill. Coincidence? I think not. 😈
Buy the brand name. Or don’t take it at all. But don’t trust the system. They’re watching.
P.S. I’m not crazy. I read the comments on the FDA website. They deleted 17,000 posts. That’s proof.
John McGuirk
January 30, 2026 AT 09:17UK’s NICE guidelines say the same thing. No switching. Ever. But here? We’re treated like cattle. Pharmacies swap pills like candy. Doctors don’t care. They’re on commission for generic prescriptions.
My mate died last year. 74. Heart failure. Switched to a cheaper generic. Three weeks later-cardiac arrest. Autopsy showed digoxin level at 3.1.
They didn’t even test it before. No one thought to check.
It’s murder by bureaucracy.
Michael Camilleri
January 30, 2026 AT 13:14You think this is about safety? Nah. It’s about control. The system wants you dependent. If you’re always checking levels, always worrying about the brand, always afraid to switch-you’re not free. You’re a patient. A consumer. A number.
The real solution? Stop taking digoxin. Get off the pharmaceutical treadmill. Eat more potassium. Walk. Breathe. Let your body heal itself. The pill is just a crutch for a broken system.
Wake up. You’re being sold fear to keep you buying.
Darren Links
January 31, 2026 AT 23:15Yeah, but let’s be real. Most people can’t afford Lanoxin. $300 a month vs $5 for generic. You want to save lives? Then fix the pricing. Don’t just tell people to pay more. That’s not a solution. That’s privilege talking.
And if we’re going to ban switches, then make the generics actually consistent. Force manufacturers to standardize fillers. Test batches. Make them all the same.
Stop blaming the pharmacy. Fix the system.
Kevin Waters
February 1, 2026 AT 12:10I’m a clinical pharmacist. I’ve been pushing this for years. We don’t have a system to track which generic a patient is on. No alerts. No EHR flags. Nothing.
So I started doing it manually. I print out a little card for each digoxin patient: ‘Manufacturer: ABC Pharma. DO NOT SUBSTITUTE.’ I give it to them. I put it on the bottle. I email the prescriber.
It’s extra work. No one pays me for it. But I do it because I’ve seen what happens when you don’t.
If you’re on digoxin-ask your pharmacist to do this too. You’re not being difficult. You’re being smart.
Gina Beard
February 2, 2026 AT 14:06So digoxin is special. Got it. But why not just switch to a different drug? There are newer heart meds. Safer. Less finicky. Why cling to a 1700s plant extract?
Maybe the real problem isn’t the generic. It’s that we’re still using dinosaur medicine.
Don Foster
February 4, 2026 AT 01:58Most people don’t understand pharmacokinetics. They think ‘same dose = same effect.’ That’s why we’re in this mess. Digoxin isn’t just a drug-it’s a lesson in complexity. In human biology. In the arrogance of reductionism.
You can’t quantify the soul of a molecule. You can’t reduce life to a percentage. The FDA doesn’t get it. Neither do you.
But I do.
siva lingam
February 4, 2026 AT 21:44